SCALEOFF – A BREIF STUDY. HOW IT WORKS?
Scaling and its causes
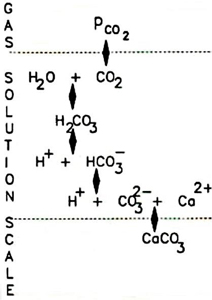
Water is a very good solvent for minerals and many other materials. All of the ionic species try to keep in a thermodynamic equilibrium with their environment, and they achieve this by combining together in clusters - perhaps growing to form crystals - or by breaking up into free ions. All of these reactions are occurring all the time as local conditions change.
Forming part of the ions calcium and carbonate, and they can form calcium carbonate - the principle scaling salt found in hard waters. We need to understand the equilibrium of these species in water, and in doing so we will know whether scaling can occur, or not. If we can, in some way, change the equilibrium with ScaleOff , we can modify the scaling behaviour of hard water.
The equilibrium solubility product for calcium carbonate is a thermodynamically defined value for a given pressure and temperature. It is the concentrations of calcium and carbonate free ions in equilibrium with a large crystal of calcium carbonate suspended in water. Equation (1) is the standard way of showing this equilibrium.
Equation 1: [Ca ++ [CO3] -> [CaCO3]
It is this equilibrium point that all waters will try and reach either by crystal growth or dissolution. At this equilibrium point, the crystal will be growing and dissolving at the same rate.
Most waters will be at equilibrium if given time, but changes in the environment, such as bore-well water emerging to the surface or the temperature and pressure changes that take place in heat-exchange plants will disturb this equilibrium. This leads to changes of the water composition. If the concentration of calcium and carbonate is greater than the equilibrium requirements, then the water attempts to reduce these concentrations and it does this by precipitation and growth of scale crystals. Equally, the water may be under saturated with respect to calcium carbonate, and this will increase the free ion concentrations by dissolving scale crystals.
The term super saturation ratio (Sr) is used as a shorthand description of the equation that determines whether the water can scale:
Sr = [Ca++]a [CO3--]a
[Ca++]eqm [CO3--]eqm
The square brackets refer to the ion concentrations (mole/litre) of the free calcium and free carbonate, the 'a' refers to actual concentrations prevalent in the water, and 'eqm' to the equilibrium concentrations as defined above.
If Sr > 1, scaling can occur; if Sr < 1, scaling cannot occur but dissolution can.
This is a fundamental, thermodynamic requirement for scale formation or dissolution. From it we can see that scaling is controlled by the equilibrium concentrations of Ca++ and CO3 ions, defined by pressure and temperature and by the actual free ion concentrations of these in any water.
All of these control factors can be modified to some degree for a given water and heat exchange plant. The one that is most readily, and economically, variable is the CO3- - ion concentration. Each reaction and ionic species demands its own equilibrium concentration. If the temperature is increased, the bicarbonate ion thermally decomposes forming carbonate
thereby increasing the super saturation ratio and providing the conditions for scale formation. It is this reaction that causes the scaling of hot surfaces - whether the surface is a kettle element or a large heat exchanger. If the pH level increases, the water tries to produce free H+ ions so as to achieve the H+/OH equilibrium for H20 One of the easiest ways it can do this is to decompose carbonic acid, and bicarbonate the ion - again producing carbonate ions and increasing the super saturation ratio.
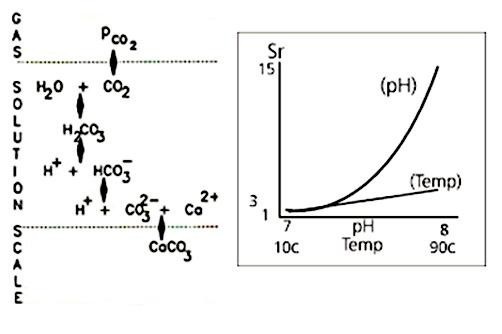
Increasing pH has a much greater effect on Sr than an increase in temperature, as illustrated in Fig 1b.
A temperature change of 80 degrees C takes a water that is close to saturated at 10 degrees C to a supersaturation ratio of between 3 and 4. This will be enough to cause severe scaling. But a change of one pH unit, from 7 to 8 increases Sr to around 15. Scaling is around 5 times as rapid - or five times as likely to occur - than under the temperature increase shown above.
pH levels and temperature are the factors that most affect the CO3 concentration, and in turn affect Sr and consequently scale formation; pH is the most dominant.
Can we then use these factors to propose, and demonstrate, a feasible explanation for the SCALEOFF water treatment? The answer is yes.
Water Treatment
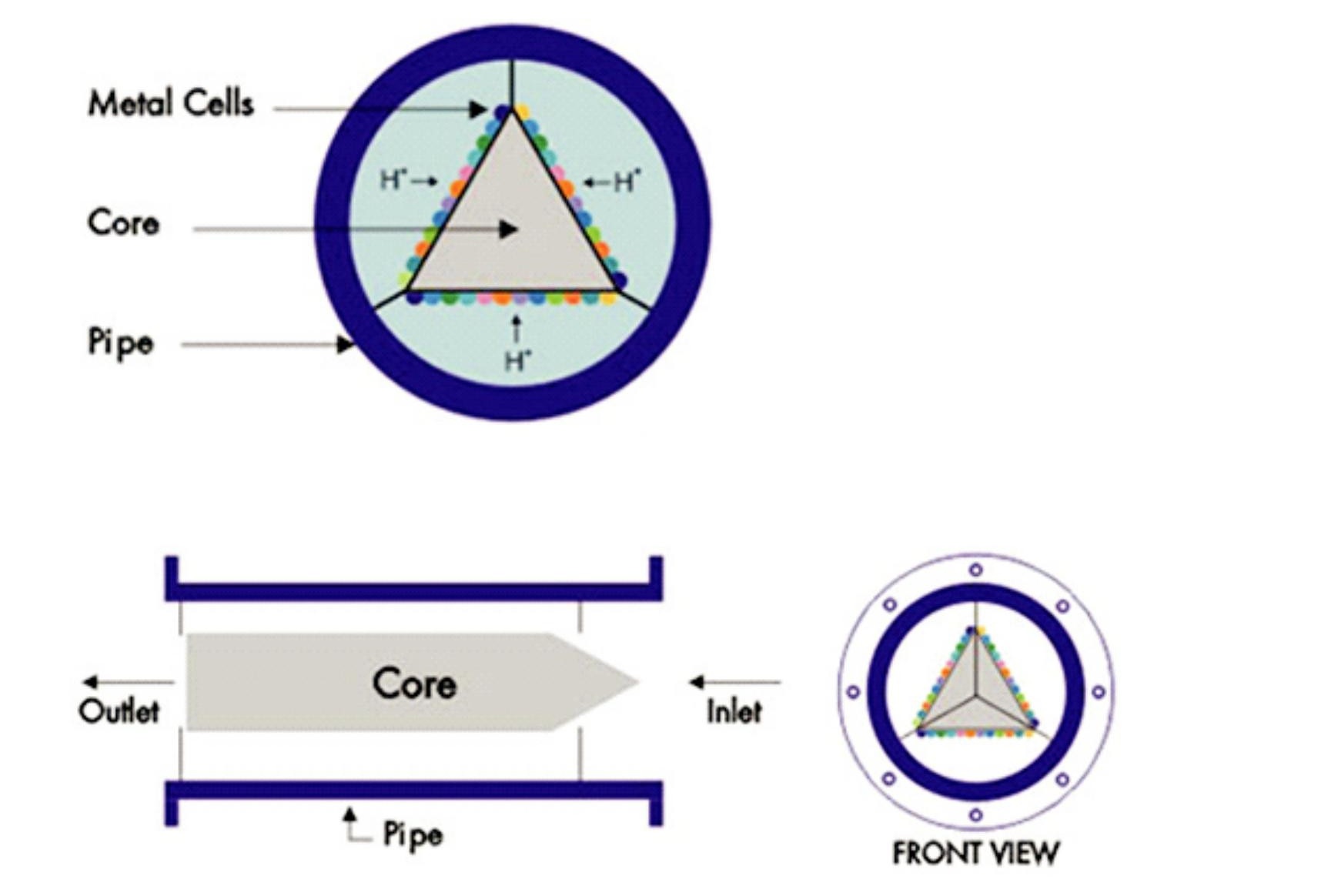
The SCALEOFFunit ( Figs 2 and 3 ), never more than 1m long, is plumbed into the system it is meant to protect. The standard unit is an apparently simple device; water is made to flow past a special alloy insert in a length of pipe.
If the unit is to work, it must change the water as it flows past. It must, in some way, reduce the supersaturation ratio of the water, i.e. the free Ca++ and free CO3-- ions must be reduced. The most obvious way would be by precipitating them as calcium carbonate - much like lime softening techniques.
Because of the composition of the unit, there are only two ways in which it can work. First, the special alloy could either adsorb ions from the solution or corrode. Either event could increase the pH locally, increase the Sr locally, and cause precipitation of calcium carbonate, thus providing the desired result that downstream of the SCALEOFF the water is part-softened and carries suspended calcium carbonate crystals. Secondly, the shape of the unit promotes turbulence; the associated pressure differences could cause dissolution of Co2 gas which may get stripped from the water when it becomes open to the atmosphere, in a cooling pump for example, and therefore reduce the total carbonic species in the water.
The second method is independent of alloy composition, but copper or zinc inserts, for example, do not work in practice. We are therefore drawn to the first, the special alloy, effect mechanism.
HOW DOES SCALEOFF PREVENT SCALING IN YOUR EQUIPMENT ?
The working principle of SCALEOFF is very simple and proven. It incorporates use of the Galvanic Principle, Chemical Characteristics of water and Fluid Dynamics. SCALEOFF exploits solubility characteristics of Calcium and Magnesium salts in water with change in its pH value. SCALEOFF locally increases the pH value of water before it reaches high temperature zone and then precipitates out the hardness causing salts in the form of colloids as water flows through SCALEOFF
As water passes through SCALEOFF, the whole core inside gets negatively charged, since water itself acts an electrolyte within the equipment. This negatively charged core attracts H+ from water, which are the lightest ions. The relationship between pH of water and H+ is expressed by the formula pH∝ 1/H+. Thus with the H+ becoming less and less, pH value of water increases, thereby precipitating hardness causing Calcium and Magnesium Salts.
There could be a doubt in mind regarding formation of scales within SCALEOFF itself and that the equipment itself might get chocked after sometime. However, this is not the case since the shape of core is trapezoidal, which creates turbulence in the water, and the scale particles being very small, the flow of water carries away these colloidal particles with it and the equipment remains completely clean forever.
As with any other equipment, SCALEOFF too has its optimal operating range, within which it performs best. Ideally, the pH value of water should not exceed 8.0 in recirculation and the minimum flow rate as is applicable for various pipe sizes should be strictly maintained. Minimum and Maximum flow rates corresponding to various pipe sizes.